Dissecting the Neurocircuitry that Modulates Binge Alcohol Drinking
The Role of Neuropeptide Y (NPY) in the Top-Down Control of Binge Alcohol Drinking
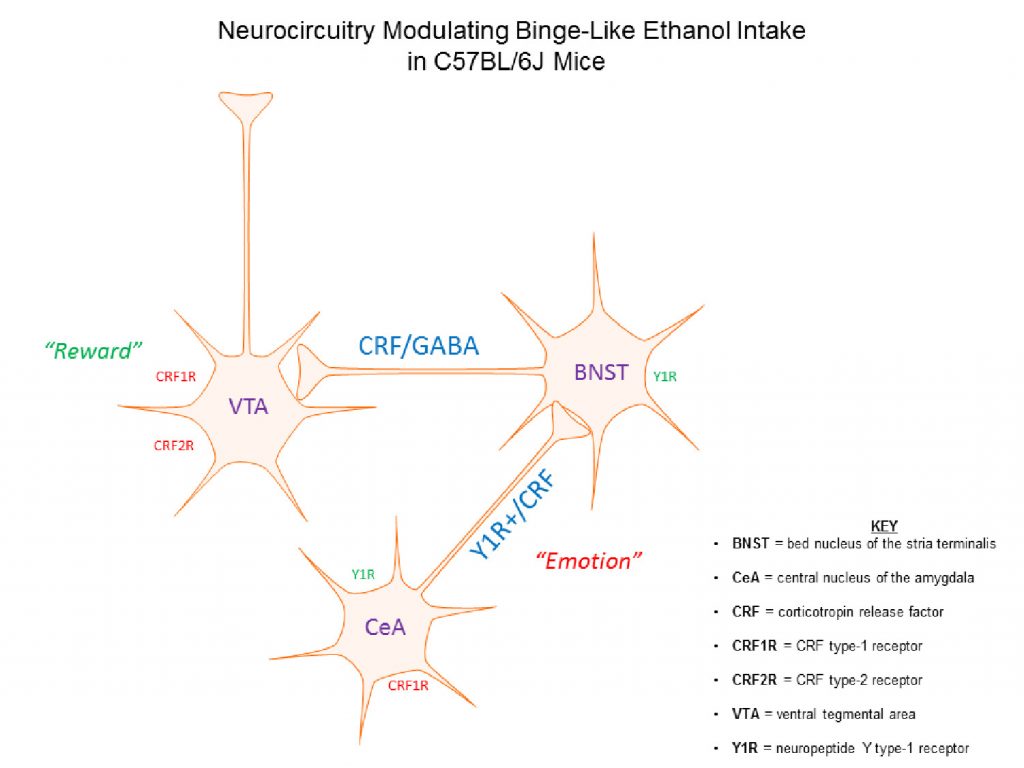
Alcohol dependence and relapse in abstinent alcoholics are major health problems throughout the world and neurochemical pathways that modulate these disorders are currently under investigation. However, the neurobiology underlying binge drinking, a dangerous pattern of behavior that proceeds and contributes to dependence, has received far less attention. Thus, it is of paramount importance to identify the neurocircuitry in the brain that modulates binge drinking as such knowledge will provide insight into the initial stages of alcohol use disorders (AUDs). We have shown that NPY signaling in regions of the extended amygdala, a set of interconnect brain regions that integrate emotional behaviors, plays a key role of modulating binge drinking in mice (Sparrow et al., 2012; Pleil et al., 2015). Binge alcohol drinking reduces NPY levels in the extended amygdala, and compounds that activate NPY type-1 receptors (Y1R) blunt binge alcohol drinking in mice when infused into the central nucleus of the amygdala (CeA) and bed nucleus of the stria terminalis (BNST). More recently we have begun to determine if NPY neurocircuitry originating in the medial prefrontal cortex (mPFC) modulates binge drinking. The mPFC provides top-down regulation of the extended amygdala, in part through a glutamatergic mPFC → basolateral amygdala (BLA) circuit that is modulated by Y1R signaling. We found that a history of binge alcohol drinking in mice attenuates NPY levels in the mPFC, analogous to what we have found in the extended amygdala. Further, a Y1R agonist and a Y2R antagonist, when individually infused into the mPFC, blunt binge drinking of alcohol, and chemogenetic silencing of Y1R+ neurons originating from the mPFC and projecting to the BLA blunts binge alcohol intake. Results from our current line of NPY studies address a critical gap in the literature regarding the role of the mPFC, NPY signaling in this region, and the functional interaction between the mPFC and extended amygdala in the modulation of binge alcohol intake in mice (Robinson et al., in press). This research is funded by the National Institute on Alcohol Abuse and Alcoholism (2R01AA013573-15A1).
The Role of Corticotropin Releasing Factor (CRF) Signaling in Binge Alcohol Drinking
We have also demonstrated the CRF signaling in the extended amygdala modulates binge alcohol drinking. We found that binge alcohol drinking increases CRF levels in CeA and that a CRF type-1 receptor (CRF1R) antagonist, when injected into the CeA, protects against excessive binge drinking in C57BL/6J mice (Lowery-Gionta et al., 2012). There is a growing body of literature demonstrating functional interactions between brain regions that integrate emotional responses (such as the extended amygdala) with those the modulate the reinforcing properties of drug and natural rewards (e.g., food and sweets), including the ventral tegmental area (VTA). Consistently, we have found that a CRF1R antagonist, and a CRF2R agonist, when infused into the VTA blunt binge alcohol drinking, and that chemogenetic silencing of a CRF circuit originating in the BNST and innervating the VTA is protective against binge drinking (Rinker et al., 2017). CRF neurons in the BNST co-express GABA, and we recently found that chemogenetic silencing of a GABAergic BNST → VTA pathway also blunts binge alcohol drinking (Companion & Thiele, 2018). These exciting finds provide novel insight that help reveal how stress systems in the brain play an influential role in motivating binge alcohol drinking by interacting with reward systems. This research is funded by the National Institute on Alcohol Abuse and Alcoholism (5R01AA022048-05).
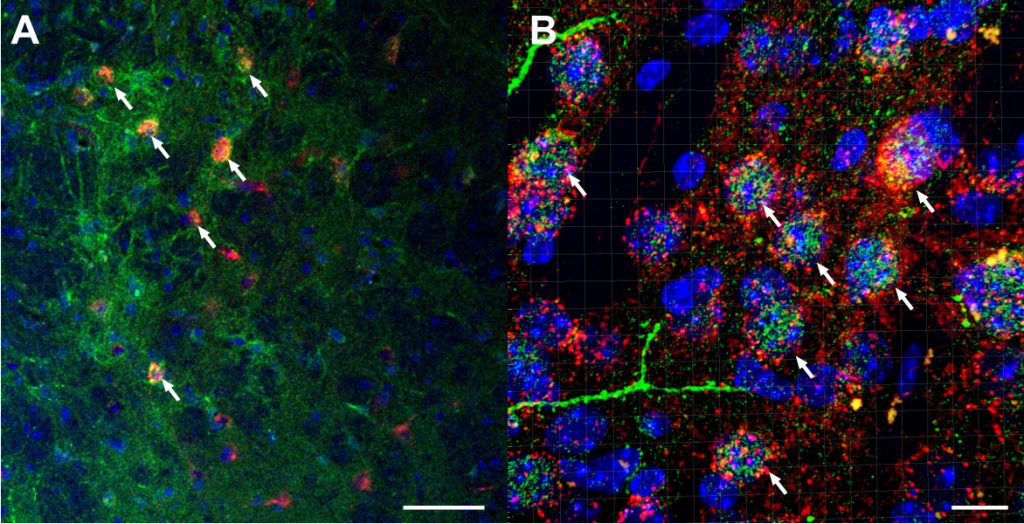
A) Confocal imaging of AAV8-hSyn-DIO-hM4d-mCherry viral vector (red) in GABAergic neurons of the BNST co-expressed with anti-CRF in Alexaflour-488 (green) and cell nuclei DAPI staining (blue) at 20x magnification. White arrows highlight co-localized neurons (scale bar = 50 µm). B) Confocal, z-stacked 3D reconstructed of co-localization of AAV8-hSyn-DIO-hM4d-mCherry virus (red) with CRF (green) and DAPI (blue) at 63x magnification using IMARIS software. White arrows highlight co-localized neurons (scale bar = 10 µm). Companion & Thiele (2018).
Bottom-Up Brainstem Circuits Modulate Binge Alcohol Drinking and the Aversive Properties of Alcohol
Considerable attention has been paid to the reinforcing effects of alcohol and how these effects motivate alcohol intake. There is strong evidence that alcohol also entails aversive effects and that these effects, because they are clearly dose related, can act as a deterrent to overconsumption. One pre-clinical behavioral assay for the aversive effects of alcohol is the development of a conditioned taste aversion (CTA). When a taste is paired with a treatment which produces aversive internal symptoms, a strong aversion to the taste develops. Studies comparing different rodent strains suggest a link between sensitivity to the aversive effects of alcohol and the propensity to voluntarily ingest alcohol. Importantly, recent data show that mice selectively bred to achieve high blood alcohol concentrations (BECs) while binge drinking exhibit reduced sensitivity to the aversive properties of alcohol without alterations in sensitivity to alcohol’s reinforcing properties. Thus, binge alcohol drinking in these mice may be driven by reduced sensitivity to alcohol’s aversive effects. Because the neurocircuitry underlying the aversive effects of alcohol is still poorly understood, we have begun to combine cutting-edged chemogenetic, molecular, and behavioral tools to characterize the neurocircuitry modulating aversive reactions to alcohol and binge alcohol consumption. Since we have previously shown that doses of alcohol that support ethanol-induced CTA activate norepinephrine (NE) brainstem regions (Thiele et al., 1996, 1997, 2000), our research focuses on NE circuits of the brainstem that provide bottom-up regulating for forebrain regions involved with alcohol consumption and which modulate the rewarding and aversive properties of alcohol. A better understanding of these ‘aversion” circuits may help identify novel ways of curbing excessive alcohol drinking. This research is funded by the National Institute on Alcohol Abuse and Alcoholism (1R01AA025809-01).
Translational Research: Probing Novel Medications for Treating AUDs
For many years our laboratory explored the modulation of alcohol consumption by brain systems that have been demonstrated to regulate feeding behaviors. One area of research focused on the melanocortin (MC) system, which is composed of neuropeptides produced in the hypothalamus. We showed that alcohol consumption reduces the normal levels of MC neuropeptides in the brain (Navarro et al., 2008), and that activation of the MC-4 receptor (MC4R) in the brain reduces alcohol drinking (Navarro et al., 2005). More recently, we showed that MC4R signaling in the lateral hypothalamus modulates binge alcohol drinking (Sprow et al., 2016). Interestingly, others have demonstrated that the drug bupropion (BUP) activates MC signaling, and when combined with the opioid receptor antagonist naltrexone (NAL) these drugs significantly reduce eating and bodyweight in humans. This research led to the development and FDA-approval of the medicine, Contrave ®, which combines BUP and NAL into one drug. Our research demonstrating a role for the MC system in modulating alcohol intake, and evidence the BUP activates the MC system, motivated a translational research program between our lab and psychiatrists in the UNC School of Medicine who are experts in clinical trial testing of medications for treating AUDs, a collaboration funded by a UNC School of Medicine/TraCS Translational Team Science Award (TTSA018P1). As part of this collaboration we recently demonstrated that BUP, alone and in combination with NAL, blunts binge alcohol drinking in mice (Navarro et al., in press). Further, an initial clinical trial study has provided evidence that combined BUP and NAL may be an effective therapy for curbing binge drinking in people, and a larger-scale phase II clinical trial study, funded by the National Institute on Alcohol Abuse and Alcoholism (5R21AA025186-02), is currently in progress. This exciting translational project may lead to the discovery of desperately needed novel medications for treating AUDs.